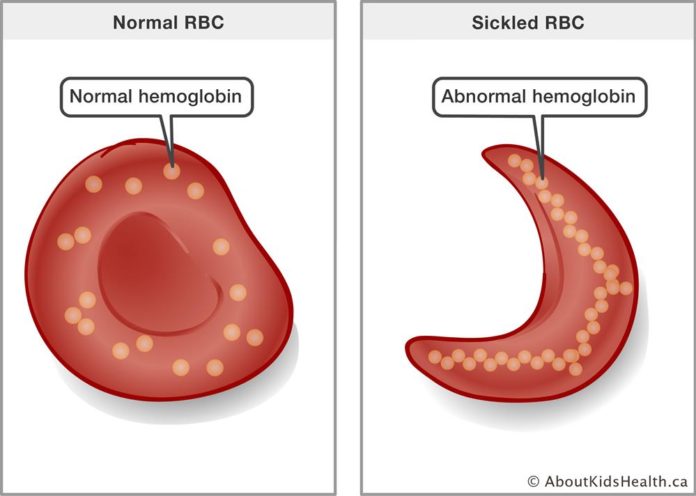
Sickle cell is an inherited blood disorder affecting approximately 100,000 Americans with sickle cell disease. Although this condition lacks a cure, it can be effectively managed through various treatment options, including medications, chemotherapy, and occasionally finding a genetic match for bone marrow. Sickle cell disease results from misshapen red blood cells, forming a sickle shape instead of the normal round shape. Symptoms include pain and anemia, typically diagnosed through newborn screenings.
While sickle cell remains incurable, it can be managed with medications and treatments that are often extremely intense. For instance, if patients are going to use chemotherapy to manage sickle cells they will be placed on a high dose of chemotherapy to eliminate the sickle cells. If the patient needs to have a bone marrow transplant, success is highly unlikely since only about 15% of patients have matched siblings and for non-related donors, the chances of a match are even lower. But now the FDA has approved a new type of treatment: Lyfgenia therapy.
Lyfgenia therapy involves using a piece of the HIV virus to deliver a functional version of the hemoglobin-producing gene. Essentially, this treatment repurposes the virus to carry and insert a healthy gene responsible for hemoglobin production into the patient’s cells. While this brings hope to those suffering from sickle cell anemia, concerns arise regarding the accessibility and affordability of the treatment, which could cost around two million dollars, posing potential challenges for treating a larger population.