No matter how trivial, it is clear that COVID-19 controlled many aspects of our lives in 2020. The pandemic was devastating, spreading rapidly throughout the world before largely overturning our daily lives in March. Although it was a bleak time, it seems that some of the control that we lost over our lives will be regained, with the rollout of a new vaccine. Although news of developed vaccines approved for use has brought us some hope, many questions are raised about the vaccine itself. What do we know about the vaccine? Is it safe, especially since it was developed so quickly? Is the government putting microchips in the vaccine? Here is a summary of the available information regarding the FDA approved vaccines that may answer those questions and more.
Why are the COVID vaccines being produced so fast?
There are currently two FDA-approved vaccines, one created by a Pfizer-BioNTech partnership and one by Moderna. The Pfizer-BioNTech vaccine is given to those aged 16+ in 2 doses over 21 days, whereas the Moderna vaccine is given to those aged 18+ in 2 doses, 28 days apart. Anyone younger than 16 will have to wait for a new or revised version of the vaccines. Both vaccines have potential side effects such as pain at the injection site, tiredness, headaches, muscle pain, chills, joint pain, and fever. The Moderna vaccine may cause swollen lymph nodes, nausea, and vomiting. Not every recipient of the vaccine will experience side effects, and those symptoms rarely last more than a few days. Thanks to the decades of research done on mRNA, which both vaccines use (and are the first vaccines using mRNA to be approved), the vaccine was able to be produced in a short amount of time, ameliorating concerns about the short vaccine rollout period. Using mRNA in vaccines uses the protein-making process in cells to trigger a response that builds an immunity to SARS-CoV-2 (the virus that causes COVID-19). In comparison, traditional vaccines use a deactivated version of the disease-causing pathogen to trigger a response, resulting in antibody creation.
Are there microchips in the vaccine?
Due to the lack of education (largely on the mRNA portion) about the vaccine, some people have been led to believe that microchips are being put into us to alter our DNA. mRNA doesn’t affect or interfere with our DNA in any way. It never enters the cell’s nucleus, and the cell breaks down the mRNA after it finishes using the instructions. None of the other ingredients contain or resemble anything microchips.
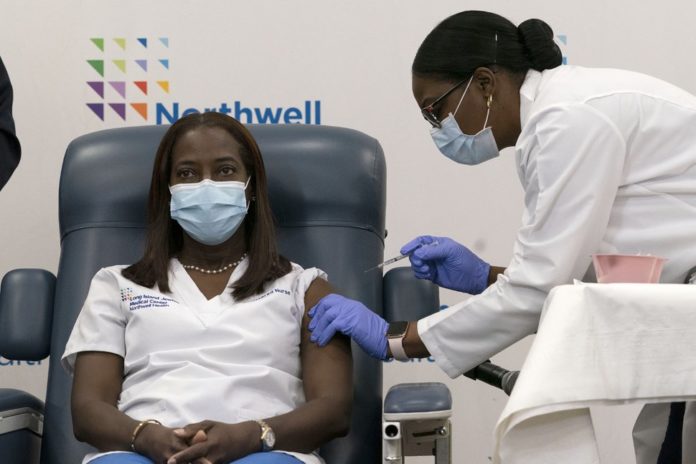
Who will receive the first doses of the vaccine? When will they be distributed?
Now that a working vaccine has been approved, questions of “when” and “who” have been brought up. With the vaccines available, it’s up to the current institutions and people in power to distribute/enforce these vaccines in an effective manner. The CDC recommends the vaccines to be distributed in the following order: Healthcare personnel and residents of long-term care facilities, frontline essential workers (including teachers) and people aged 75 and older, people aged 65-74, people aged 16-64 with underlying health conditions, and other essential workers. Early recipients of the vaccine have also included politicians, some of whom did not believe in COVID-19 until it was too late. These recipients who’ve been receiving the vaccines are being criticized for protecting themselves but not the people they serve. As Pfizer-BioNTech and Moderna manufacture more of the limited vaccines, we can be more flexible with the order of prioritization. Additionally, it is important to be cautious as people begin to receive the vaccine. After vaccination, the CDC recommends continuing to wear a mask and practice social distancing. As we enter 2021, we will have to be patient and considerate of others so that we can not only slow down the virus but also work towards eliminating it.